Understanding the Clinical Trial Landscape and the Role of Artificial Intelligence
The clinical trial market is massive. ClinicalTrials.gov currently lists more than 520,000 clinical research studies on its site. But clinical trial design and execution continue to face several challenges, and innovative solutions are needed. The drug development process is notably time-intensive and costly. To bring a new drug to market, it takes 10-15 years and an estimated $314 million to $4.46 billion in research and development costs. In traditional clinical trials, other substantial hurdles exist, including 23% of trials failing to achieve planned recruitment timelines and late-stage studies experiencing average dropout rates of 19%. These pressures often require timeline extensions and additional study sites, which further delays patient access to potentially life-saving treatments.
The need for AI in clinical trials is understated. The applications of AI in clinical trials span the research lifecycle from protocol design and patient recruitment to real-time data analysis and safety monitoring. While AI offers opportunities for efficiency and innovation, new challenges are introduced related to data privacy, regulatory compliance, and implementation complexity. Understanding these benefits and challenges is crucial for organizations seeking to leverage AI effectively in their clinical research programs.
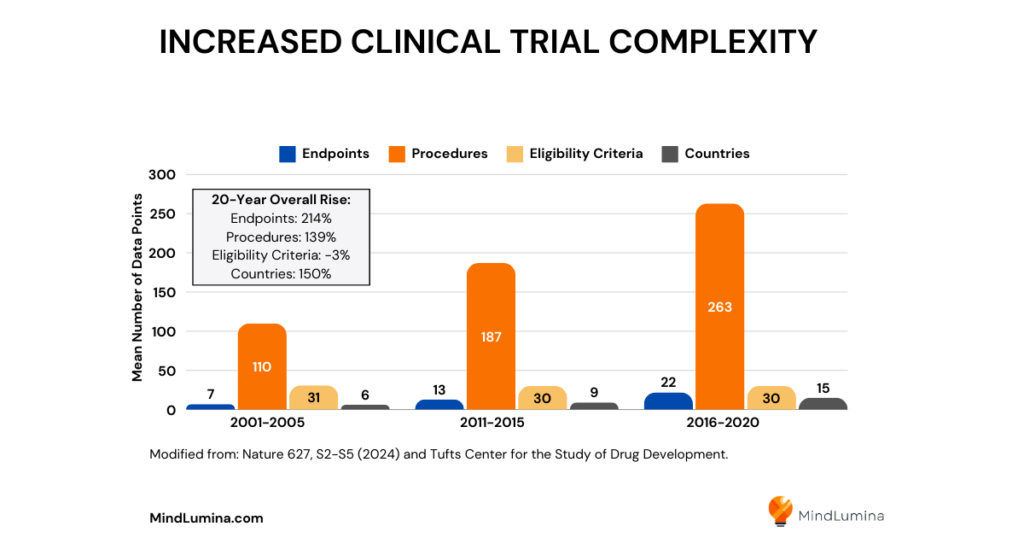
Increased Clinical Trial Complexity
The complexity of modern clinical trials further intensifies these challenges. A 2021 study found that Phase 3 clinical trials generate an average of 3.6 million data points – tripling the amount collected 10 years prior. This surge in data complexity stems from increasingly sophisticated trial designs, biomarker integration, genetic data stratification, and the growing focus on rare diseases. More countries are conducting clinical trials than ever before, and study protocols are requiring more endpoints, procedures, and data points.
AI in FDA Discussion Papers and Draft Guidances
The use of artificial intelligence (AI) to improve and expedite clinical trials is quickly gaining momentum. In May 2023, the US Food and Drug Administration (FDA) published a discussion paper on the use of AI and machine learning in drug development, recognizing the potential of AI and its critical role in advancing research. Recently, in January 2025, the FDA issued two draft guidance documents concerning AI: one on the use of AI in the product life cycle of drugs and biologics, and the other on AI-enabled devices.
Applications of AI in Clinical Trials
AI-Optimized Protocol and Clinical Trial Designs
The use of AI and machine learning in clinical trials has changed how trial protocols are developed and optimized. AI algorithms can analyze vast amounts of electronic health records and claims data to inform more efficient study designs. By analyzing historical data, including detailed medical histories and past trial outcomes, advanced AI models can identify optimal patient populations, sample sizes, endpoints, and treatment regimens for studies.
Given the increasing complexity of clinical trial protocols, AI’s capacity to analyze historical protocol data and predict potential design flaws before implementation represents a significant advancement in protocol optimization.
This optimization is particularly crucial based on a study reporting that 57% of clinical trial protocols required at least one substantial amendment, 45% of these amendments being avoidable, and costs of $535,000 per Phase 3 protocol amendment.
AI-powered platforms can be valuable in preventing costly protocol amendments by designing more intelligent studies. For example, the Trials.ai platform (acquired by ZS) uses natural language processing and machine learning to create clinical study documents with digitization and automation. And, Phesi’s Trial Accelerator uses a clinical development database of over 70 million patients and real-world data to optimize protocol development and execution. Both AI platforms enhance study performance, reduce errors, and minimize manual input, further improving efficiency and accuracy in the research and development process.
Digital Twins Simulations
Digital twins technology creates AI-powered patient simulations based on patients’ medical histories, genetic profiles, and real-time health information. These virtual patient models can simulate disease progression and treatment responses, allowing researchers to test multiple scenarios, predict outcomes, and identify potential challenges.
Unlearn, an AI company based in San Francisco, has worked in collaboration with Johnson & Johnson Innovative Medicine and Abbvie on digital twin studies. Recent studies presented at the Alzheimer’s Association International Conference 2024 demonstrated that digital twins reduced control arm sizes by 33% in Phase 3 trials and reduced overall sample sizes by 10-15% in a Phase 2 trial. In Alzheimer’s research, faster enrollments and shorter trial durations could translate into millions of dollars in cost savings.
Accelerated Patient Recruitment and Matching
Advanced technologies now leverage AI for recruitment in clinical trials by dramatically accelerating the identification of potential trial participants, improving clinical trial matching, and predicting where eligible patients are likely to be located.
Using large language models (LLMs), the National Institutes of Health (NIH) developed an AI algorithm called TrialGPT that not only improved patient matching to clinical research studies listed on ClinicalTrials.gov but also helped clinicians spend 40% less time on screening while maintaining accuracy.
Software made by Deep 6 uses AI and natural language processing in optimizing eligibility screening for clinical trials by rapidly analyzing millions of medical records, including unstructured data such as physician notes and lab reports. It identifies suitable participants in clinical trials within minutes rather than months. The impact is remarkable – for example, when Texas Medical Center implemented these AI-powered matching tools, they identified 80 potential participants in minutes compared just 12 patients in the previous six months through traditional methods.
These AI solutions can evaluate complex eligibility criteria more efficiently, ultimately accelerating enrollment in clinical trials and advancing medical research at an incredible pace.
Streamlined Site Selection and Optimization
Traditional site selection challenges have historically resulted in substantial costs, with research indicating that 37% of sites underenroll and 11% of sites fail to recruit any patients for clinical trials. AI-powered solutions are now addressing these challenges by analyzing diverse data sources, standardizing inconsistent information across sites, optimizing the process of identifying and selecting optimal research sites, and improving study start-up efficiency.
For example, AI tools like ICON’s One Search have shown a 53% reduction in median time for site identification and a 50% decrease in non-enrolling sites. These impressive metrics demonstrate the great impact of AI-driven site selection strategies.
In overcoming traditional obstacles, AI can also identify suitable investigators based on their experience, performance history, and patient populations. Machine learning models can analyze historical site performance data to predict potential challenges and recommend proactive solutions, significantly reducing the risk of study delays. Additionally, AI systems can continuously monitor site performance throughout the trial, enabling research teams to make data-driven decisions about resource allocation and site support, leading to reduced study delays, improved cost efficiency, and better overall trial outcomes.
Personalized Patient Engagement and Outreach Strategies
AI tools integrated with digital health technologies have modernized patient engagement in clinical research, enabling continuous communications with trial participants through wearables, smartphone apps, and chatbots.
The smartphone-based app by AiCure illustrates how the integration of AI and use of digital health technology can successfully work together. This app has features such as dosing support, two-way messaging, and can analyze digital biomarkers from patients’ facial expressions, body movements, and voice patterns.
Another company, AllazoHealth, analyzes patient data including prescription records, medical claims, and behavioral patterns to develop personalized outreach strategies. For each patient, the best message content, format, delivery platform, and timing is identified. This tailored approach ensures that trial-related communications resonate and remain informative, engaging, and motivating throughout the trial.
These AI technologies aim to improve the patient experience with clinical trials through personalized approaches that fit easily into participants’ daily routines. Especially in decentralized clinical trials, AI makes trial participation more accessible and manageable for patients.
Improved Clinical Trial Diversity and Inclusion
In 2020, the participation of racial and ethnic minorities in Phase 3 oncology trials supporting US FDA filings was alarmingly low – only 8% of participants were Black, 6% Asian, and 1% Hispanic. In 2022, in an effort to improve the enrollment of historically underrepresented populations, the FDA issued guidance requiring Diversity Action Plans for clinical studies, with the need for sponsors to define these enrollment goals early in the clinical development process.
Open source AI tools like Trial Pathfinder demonstrate how AI for clinical research can effectively support these diversity initiatives. This AI framework from Stanford uses real-world data from electronic health records to set eligibility criteria for clinical trials. To address underrepresented populations in cancer research, a collaborative research study with Genentech showed that relaxing eligibility criteria could double the number of eligible patients across diverse demographics, including African Americans, women, and older patients.
These AI-driven approaches are successfully bridging patients to clinical trials, utilizing real-world data from electronic health records to broaden access to clinical trials, benefiting underserved populations that the FDA guidance aims to include.
Improved Patient Adherence and Retention
Adherence during the clinical trial process is a valid concern for study sponsors. Negative outcomes can include patient dropouts, delayed timelines, increased recruitment costs, and study failure. The use of AI can improve patient adherence to study protocols. AI-embedded wearables and sensors facilitate remote monitoring of patient health status, while automated alerts and reminders delivered through smartphones help track medication compliance and missed appointments. These technologies are particularly valuable in decentralized clinical trials, where they enable participants to engage in studies from home, substantially reducing the burden of frequent in-person visits and making trial participation more convenient and less disruptive to daily life. By providing real-time information and addressing participant concerns promptly, these AI-powered solutions help maintain higher retention rates while ensuring protocol compliance throughout the duration of the trial.
Continuous Clinical Trial Safety Monitoring
The use of AI in clinical trials facilitates safety monitoring by enabling continuous or frequent real-time measurements of patient health data through digital health technologies and activity trackers. The integration of AI with wearable devices, mobile apps, and other digital health platforms create systems that can track vital signs, activity levels, sleep patterns, and other health indicators of a patient’s well-being during the duration of a trial. This advanced monitoring allows for immediate detection of potential safety signals and adverse events.
Generative AI platforms, such as those developed by Saama, actively monitor patient safety by automatically identifying clinically significant issues, early warning signs, and potential adverse events before they become serious concerns. By analyzing multiple data points – including medication adherence, reported side effects, and appointment attendance patterns – these advanced AI algorithms can predict potential safety risks, allowing research teams to intervene proactively.
AI can prevent early trial withdrawals by signaling timely interventions when participant distress or non-compliance are detected.
AI-Powered Data Collection and Management
As researchers grapple with vast volumes of structured and unstructured clinical data from various sources, AI can also automate data management tasks with incredible speed and accuracy. AI can standardize data formats across multiple sites, identify missing or inconsistent data points, reconcile information across different documents, and analyze complex datasets.
AI can help expedite data cleaning, as demonstrated during Pfizer’s COVID-19 vaccine trials. During this time when speed was especially critical, Saama’s AI-powered Smart Data Query (SDQ) technology processed data from over 40,000 participants in just 22 hours – a dramatic improvement from the traditional 30-day timeline.
This advancement in information management empowers research teams to accelerate the path from data collection to meaningful insights, ultimately enhancing both operational efficiency and the quality of trial outcomes.
Real-Time Predictive Analytics and Adaptive Trial Designs
AI can also be used to power adaptive trial designs through predictive analytics, enabling real-time adjustments to study parameters such as patient cohorts, dosages, and trial duration based on interim analyses. These intelligent systems can forecast trial outcomes early in the study, allowing researchers to make data-driven modifications that optimize resource allocation and minimize unnecessary patient exposure to ineffective treatments. Through sophisticated algorithms analyzing real-time patient data, adaptive trials can identify responsive subpopulations and adjust treatment arms accordingly, leading to more personalized therapeutic approaches and improved success rates. The FDA continues to evaluate AI applications in adaptive clinical trial designs, aiming to provide guidance to the medical community on how to best implement these approaches.
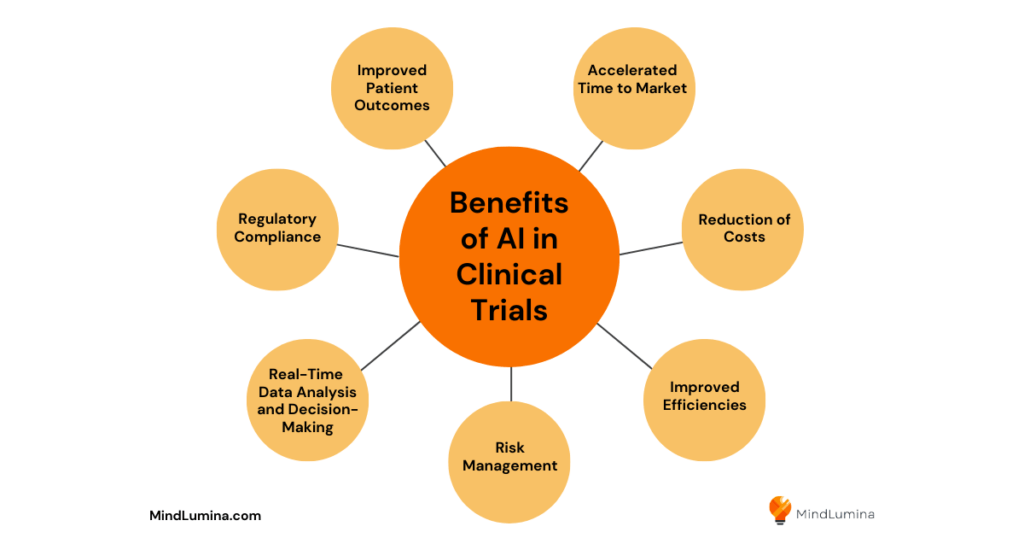
Benefits of Using AI in Clinical Trials
Improved Patient Outcomes
One of the most important benefits of AI in clinical trials is its ability to enhance treatment effectiveness and safety monitoring for participants. Through continuous data analysis and real-time monitoring, AI can detect potential adverse events earlier and enable faster interventions to protect patient well-being.
The technology’s capacity to identify optimal patient subgroups and predict treatment responses leads to more personalized therapeutic approaches. AI algorithms can analyze vast amounts of patient data to identify subtle patterns that might indicate early treatment response or potential complications. These insights enable research teams to make proactive adjustments to treatment plans, improving clinical outcomes for trial participants.
Accelerated Time to Market
AI technologies significantly compress the traditional timeline for bringing new drugs to market by streamlining multiple aspects of the clinical trial process. Advanced algorithms can reduce protocol design time from months to weeks, while AI-powered recruitment tools can cut enrollment periods.
Automated data collection and analysis systems can process information in real-time, eliminating delays associated with manual data review and validation.
AI has the ability to predict trial outcomes early, which can help sponsors make faster decisions about trial continuation or termination. By enabling faster data analysis and real-time monitoring, these technologies help sponsors meet critical milestones more quickly, potentially reducing the overall time to patient availability.
Reduction of Costs
The implementation of AI in clinical trials presents substantial opportunities for cost reduction across the entire research process.
By optimizing protocol design, improving site selection, and accelerating patient recruitment, AI helps avoid expensive protocol amendments and reduces the number of non-performing sites.
The automation of data management and the monitoring of tasks can significantly decrease operational costs.
AI-driven predictive analytics can help prevent costly trial failures by identifying potential issues early in the process.
The reduction in manual labor and improved resource allocation further contributes to overall cost savings throughout the trial lifecycle.
Improved Efficiencies
Integrating AI into clinical trial processes dramatically enhances operational productivity and resource utilization. The efficiency of clinical trial execution improves through AI’s ability to automate routine tasks, standardize processes across sites, and enable real-time data validation. These technological capabilities reduce manual workload, minimize data entry errors, and accelerate decision-making processes, allowing research teams to focus on more strategic activities.
AI systems can simultaneously process multiple data streams, enabling parallel processing of different trial aspects that would traditionally be handled sequentially. AI’s capacity to learn and adapt from experience means that efficiency gains continue to improve over time as the system processes more data.
Risk Management
AI continuously monitors clinical trial data for safety signals and potential protocol deviations, enabling proactive risk mitigation strategies. The technology can analyze patterns across multiple data sources to identify potential safety concerns before they become serious issues. By providing early warnings and predictive insights, AI helps research teams maintain trial integrity while ensuring participant safety throughout the study duration. Machine learning algorithms can detect subtle patterns that might indicate emerging risks, allowing for early intervention. The system’s ability to learn from historical data helps prevent similar issues from occurring in future trials.
Real-Time Data Analysis and Decision-Making
The power of AI transforms how clinical trial data is analyzed and utilized for decision-making purposes. Through advanced analytics capabilities, AI can process vast amounts of structured and unstructured data in real-time, providing immediate insights to research teams. This rapid analysis enables adaptive trial designs and allows for quick, data-driven decisions that can optimize trial outcomes and resource allocation.
AI can effectively analyze multiple data streams, identifying correlations and patterns that might be missed by traditional analysis methods. AI-powered dashboards provide real-time visualizations of trial progress and performance metrics, enabling faster and more informed decision-making.
Regulatory Compliance
AI technology enhances regulatory compliance by maintaining consistent documentation and ensuring adherence to protocol requirements across all trial sites. Automated systems can track regulatory submissions, monitor protocol deviations, and generate comprehensive audit trails that meet regulatory requirements. These capabilities help sponsors maintain compliance with evolving regulatory frameworks while reducing the risk of regulatory findings during inspections.
AI-powered systems can automatically flag potential compliance issues and suggest corrective actions before they become significant problems. The ability to maintain detailed documentation of all trial activities helps demonstrate compliance during regulatory audits and inspections.
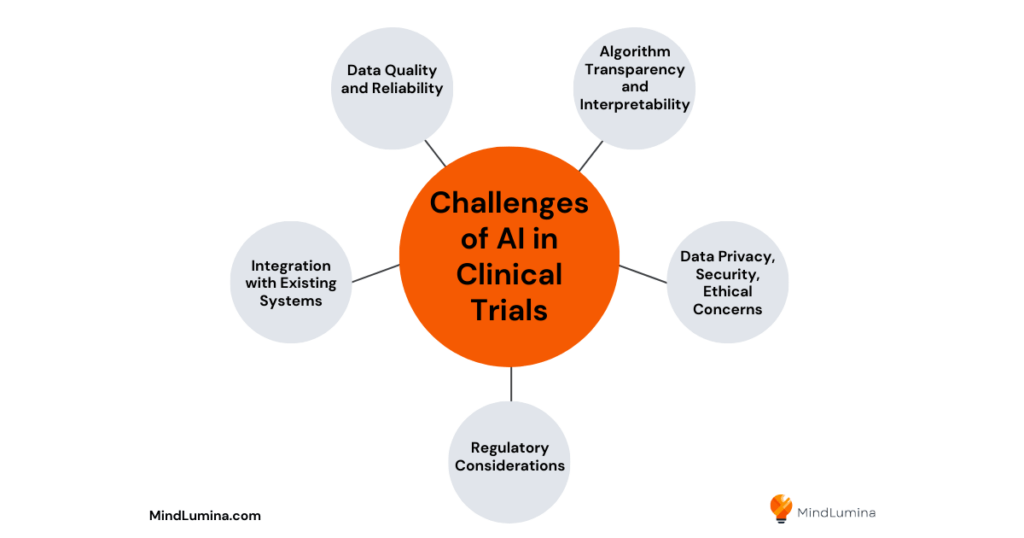
Challenges of AI in Clinical Trials
Data Quality and Reliability
The effectiveness of AI systems in clinical trials heavily depends on the quality and completeness of input data, which remains a significant challenge in the field. Missing data, inconsistent formatting across different sites, and variations in data collection methods can impact the accuracy of AI algorithms and their resulting predictions. Poor data quality can lead to biased results and unreliable insights, potentially compromising trial outcomes and patient safety.
The lack of standardization across healthcare systems further compounds these challenges. The need for extensive data cleaning and validation processes can offset some of the efficiency gains promised by AI implementation.
Algorithm Transparency and Interpretability
Understanding how AI algorithms arrive at their conclusions poses a major challenge for clinical researchers and regulatory bodies. The complexity of deep learning models often makes it difficult to trace the reasoning behind specific recommendations or predictions. This lack of transparency can create hesitation among healthcare professionals to rely on AI-driven insights, especially in critical decision-making scenarios that directly impact patient safety and trial outcomes.
The challenge becomes even more significant when explaining AI decisions to patients and regulatory authorities.
The need for interpretable AI models must be balanced against the potential loss of predictive power that might come with simpler, more transparent algorithms.
Data Privacy, Security, and Ethical Concerns
The responsible use of AI in clinical trials requires careful consideration of patient privacy, data security, and ethical implications. As trials collect increasingly sensitive personal and health information through various digital platforms, ensuring robust data protection while maintaining data utility becomes increasingly complex.
The challenge extends beyond technical security measures to include ethical considerations about data ownership, informed consent in the age of AI, and the potential for algorithmic bias affecting traditionally underrepresented populations.
International data transfer regulations and varying privacy laws across jurisdictions further complicates data handling and sharing. The need to balance data accessibility for AI analysis with privacy protection creates ongoing challenges for trial sponsors and researchers.
Regulatory Considerations
The rapid advancement of AI technologies in clinical research creates challenges for regulatory frameworks that must evolve to address new methodologies while maintaining established standards for patient safety and data integrity. Regulatory bodies face the complex task of developing guidelines that balance innovation with risk management, particularly in areas such as adaptive trial designs and real-time data analysis.
The lack of harmonized international regulations for AI use in clinical trials also makes it more difficult for sponsors conducting multi-regional studies. Different interpretations of AI validation requirements across regulatory agencies can create confusion and delays in trial approval processes. The evolving nature of AI technology means that regulatory frameworks must remain flexible while ensuring adequate oversight of AI applications in clinical trials.
Integration with Existing Systems
Incorporating AI solutions into established clinical trial infrastructure presents significant technical and operational challenges. Legacy systems, varying technical capabilities across trial sites, and resistance to workflow changes can impede the successful implementation of AI technologies.
The need for substantial investment in infrastructure upgrades, staff training, and system validation often creates barriers to adoption, especially for smaller research organizations and trial sites.
Integration challenges are further complicated by the need to maintain data integrity and system security during the transition to AI-enabled processes. The requirement for seamless interoperability between AI systems and existing clinical trial management software is paramount to the integration process.
Companies Using AI to Advance Clinical Trials
Several companies are using AI to drive innovation and provide AI-driven solutions to clinical trial optimization, patient enrollment and retention, data management, and analytics.
Key companies among them are:
- Trials.ai (acquired by ZS)
- Phesi
- Unlearn
- Deep 6
- ICON
- AiCure
- AllazoHealth
- Saama
The Future of AI in Clinical Trials
The impact of AI on clinical trials continues to evolve rapidly, promising to transform how medical research is conducted in the coming years. The integration of AI in drug discovery and clinical trial processes is expected to accelerate, with refined algorithms becoming increasingly efficient at analyzing complex trial data and predicting therapeutic outcomes with greater accuracy.
As precision medicine becomes more prevalent, AI will play a crucial role in identifying optimal patient populations and personalizing treatment approaches based on genetic, environmental, and lifestyle factors. The ability of AI to transform traditional clinical trial methods is likely to lead to more adaptive, efficient, and patient-centric study designs. These advances in precision medicine, coupled with AI’s analytical capabilities, will enable researchers to design trials that better account for individual patient variations and response patterns, potentially increasing success rates in later-stage trials.
Moreover, the next-generation of AI tools is expected to further advance clinical trial processes. The integration of AI with Internet of Things (IoT) devices and wearable technologies will enable more comprehensive real-time patient monitoring and data collection, leading to more nuanced understanding of treatment effects. In addition, the continued advancement of AI capabilities in natural language processing and computer vision is expected to improve the analysis of unstructured medical data – from medical imaging to patient narratives and clinical notes.
As these technologies mature and become more widely adopted, we can expect to see a fundamental shift in how clinical trials are designed, conducted, and monitored, leading to faster development of more effective treatments for patients worldwide.
Frequently Asked Questions (FAQs)
How can AI help in clinical trials?
AI can accelerate clinical trials by optimizing patient recruitment, improving trial design, identifying potential safety concerns, monitoring participant adherence, predicting treatment responses, analyzing large volumes of complex data, and improving data quality. All of these AI applications can greatly enhance the speed and efficiency of the clinical trial process.
How is generative AI used in clinical trials?
The role of generative AI in clinical trials includes automating protocol development, creating patient education materials, simulating trial scenarios, generating synthetic data for trial planning, and predicting patient outcomes. These capabilities help streamline trial design, enhance patient engagement, and accelerate the research process.
What is the AI in clinical trials market size and its projected growth?
The global AI in clinical trials market size was USD 1.58 billion in 2023, estimated to be USD 2.04 billion in 2024, and projected to grow to USD 20.16 billion by 2033, at a compound annual growth rate (CAGR) of 29% from 2024 to 2033.